47+ Solution Examples Chemistry
Solution Examples Chemistry. Image result for molality vs molarity solution examples chemistry teacher life. The molecules of a solution are evenly distributed throughout the solution.

A soda is a saturated solution of carbon dioxide in water. 15 rows examples of environmental aqueous solutions include rainwater, seawater, and groundwaters. Solid solutions generally involve melting two or more solids at high temperatures, mixing, and then letting them cool and solidify.
simple embroidery designs easy salon de jardin table ronde 8 personnes sigma 150 500mm nikon compatibility sol pvc imitation jonc de mer
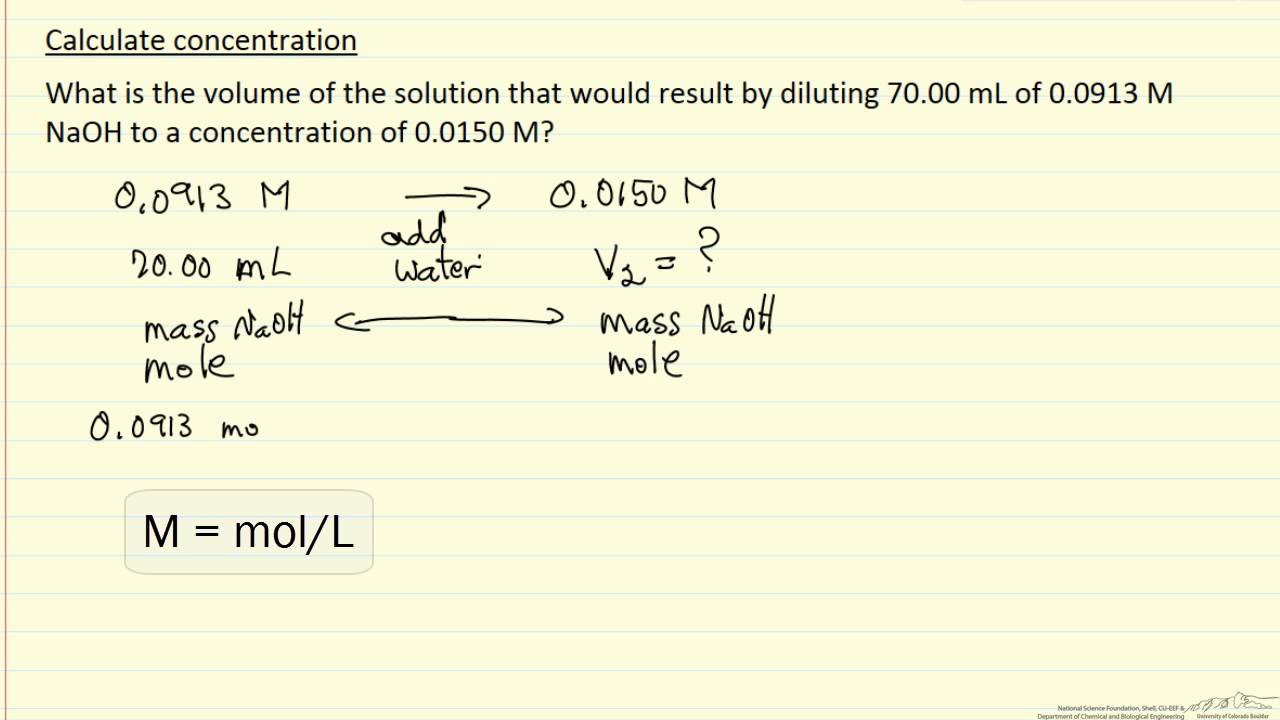
Calculate Concentration (Example) YouTube
The dispersed phase for the above mentioned examples is liquid and the dispersion medium is liquid as well. Also, the solvent does not need to be water. A solution is a homogeneous mixture of one substance dissolved in another. A soda is a saturated solution of carbon dioxide in water.
Also, the solvent does not need to be water. Examples of solutions include water vapor in air, table sugar in water, steel, brass, hydrogen dissolved to palladium, carbon dioxide in water and ethanol in water. There are many examples of solid/liquid solutions in everyday life. For example, salt in water, carbon dioxide in carbonated beverages, water vapor. Here are some.

This is termed as a solid aerosol. 7 rows any two substances which can be evenly mixed may form a solution. The dispersed phase for the above mentioned examples is solid and the dispersion medium is gas. Air, for example, is a solution consisting chiefly of oxygen and nitrogen with trace amounts of. A soda is a saturated solution of.

The term solution is commonly applied to the liquid state of matter, but solutions of gases and solids are possible. A topical antibiotic solution is 1.0% (m/v) clindamycin. For example, salt in water, carbon dioxide in carbonated beverages, water vapor. Discover the parts of a solution and see examples. This is why, when the pressure is.

The dispersed phase for the above mentioned examples is liquid and the dispersion medium is liquid as well. A topical antibiotic solution is 1.0% (m/v) clindamycin. Some examples of percent compositions, their meanings, and possible conversion factors are shown in the table below: The term used for these are an emulsion. Periodic table, balancing chemical equations, writing balanced equations, stoichiometry,.
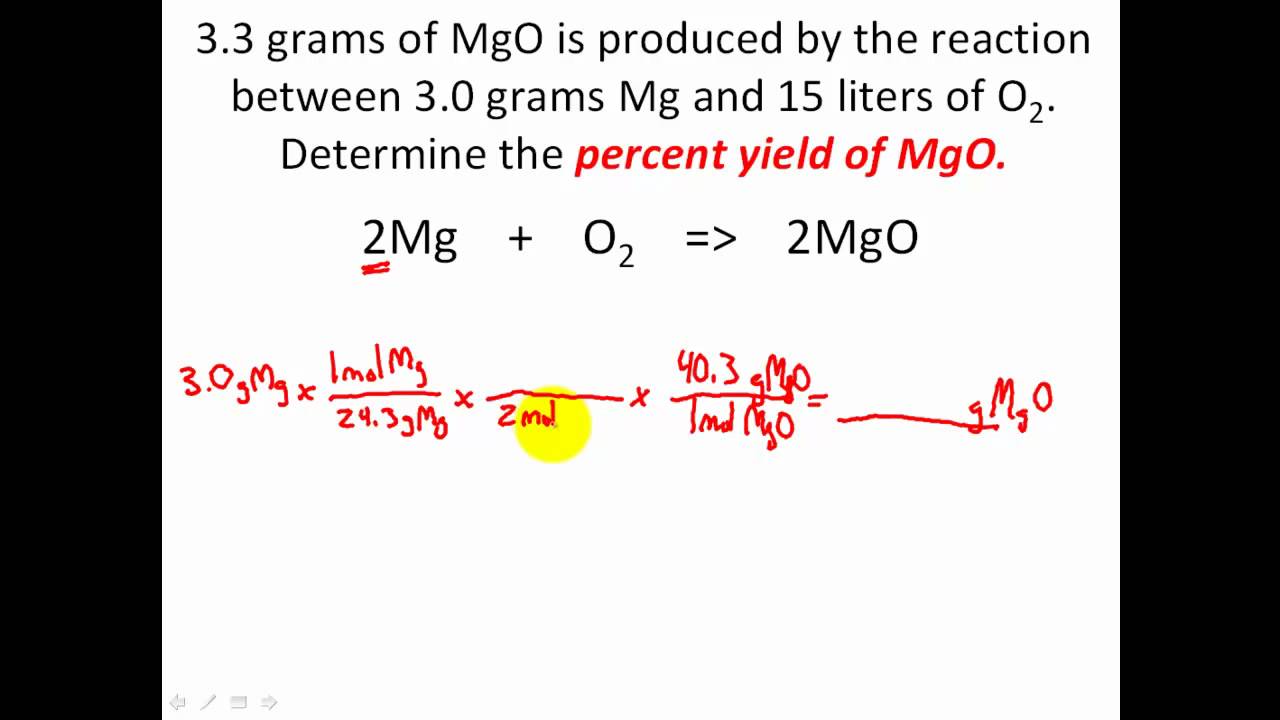
Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to what is called the limit of solubility. The molecules of a solution are evenly distributed throughout the solution. For example, salt in water, carbon dioxide in carbonated beverages, water vapor. Stoichiometry problems solved moles problem solving solving chemistry. A.